Abstract
Introduction: The survival for acute myeloid leukemia (AML) remains suboptimal. The development of immunotherapies such as chimeric antigen receptor (CAR) T cells, bispecific T cell engagers and antibody drug conjugate (ADC) therapies is dependent on the identification of novel cell surface targets. In this study, we investigated the AML plasma membrane proteome (surfaceome) in an unbiased fashion through a multi-omic data integration of proteomic and transcriptomic data.
Methods: Label-free, nonaffinity-purified enrichment of cell surface proteins and liquid chromatography-mass spectrometry (LC-MS) analysis were performed. We generated surfaceome MS data for 9 pediatric AML (pAML), 6 pediatric normal bone marrow (pND), 40 adult AML (aAML) and 13 healthy adult donors of G-CSF mobilized peripheral blood stem cells (aND). Sixteen MOLM-14 cell line samples served as internal controls. We established a customized protein sequence database building on short read RNA-sequencing using a subset of the primary samples, including 3 pAML, 4 pND, and 22 aAML. To construct the customized protein reference database, we separated identified transcripts into two categories: (i) novel and (ii) annotated in GENCODE. For transcripts annotated as "basic protein coding" in GENCODE v39, we directly fetched coding sequence from genome sequences and translated to protein sequence with standard genetic codes. For other GENCODE annotated transcripts and identified novel transcripts, we obtained their protein sequences from their predicted longest ORFs.
MS data were processed with MSFragger then further analyzed in the MOVE package. The noise reduction threshold was set to > 65% threshold in at least one searching group. Protein level quantification was evaluated using unique peptide abundances. Label-free quantitation intensity values were log2 transformed and normalized by subtracting the median. Data were imputed using bpca in the pcaMethods package and batch effects were corrected using the ComBAT method in the sva package. Protein differential expression analysis was performed using the Limma package. Based on proteomic data, differentially upregulated proteins were defined as having a P-value < 0.05, log fold change > 1 and false discovery rate < 0.1. Surface proteins were determined using The Cancer Surfaceome Atlas database (TCSA) (n=3,567).
Results: Median ages of the aAML, pAML, and pND were 60.5, 11.0, and 12.5 years. Ages of aND were missing. The median blast percentages of aAML and pAML were 68.5% and 80.0%. FLT3 ITD, NPM1c and mutant CEBPA were present in 9 aAML, 9 aAML and 3 aAML, respectively, and were absent in all pediatric samples.
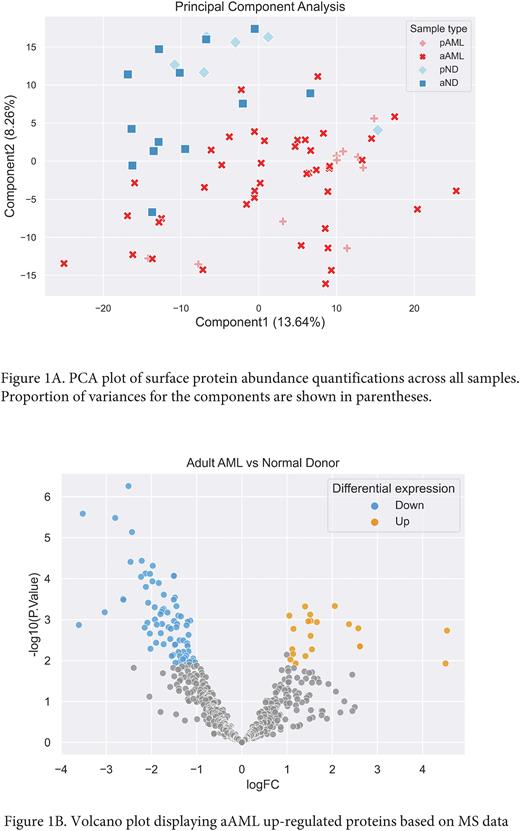
The customized RNA-transcript generated FASTA database with complemented UniProt isoforms was used to search all proteomic data sets from all 84 samples. A total of 3652, 3224, 3409, and 3180 proteins were identified in aAML, aND, pAML and pND samples. After crosschecking in TCSA knowledge base, 670, 611, 612, and 578 surface proteins were identified in aAML, aND, pAML, pND samples. Principal component analysis (PCA) revealed overlap of pediatric and adult samples, while normal donor samples shown little overlap with AML samples (Figure 1A). When compared with pND samples, pAML samples revealed 66 uniquely and 28 differentially up-regulated surface proteins. When compared with aND samples, 60 uniquely and 21 differentially up-regulated surface proteins were found in aAML (Figure 1B). Comparison between the aAML and pAML detected 59 and 1 uniquely expressed surface proteins in aAML and pAML samples, respectively.
Conclusions: These data represent an unbiased approach to antigen discovery in both adult and pediatric AML. Future studies will include extending RNA-seq data to additional samples of pediatric AML and normal donors to develop a more comprehensive protein search library, coordinated-analyzing transcriptomic and proteomic data by patient demographic and genetic subgroups, and functional validation of novel targets.
Disclosures
Gill:Asher Bio: Research Funding; Hemogenyx: Honoraria, Research Funding; Immpact Bio: Honoraria; Astra Zeneca: Honoraria; Interius: Current holder of stock options in a privately-held company, Research Funding; Mission Bio: Membership on an entity's Board of Directors or advisory committees; Carisma: Current holder of stock options in a privately-held company, Research Funding; Novartis: Patents & Royalties, Research Funding. Bernt:Merck: Other: Husband is an employee of Merck and has stock; Epizyme: Patents & Royalties; Syndax: Research Funding. Xing:Panorama Medicine: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal